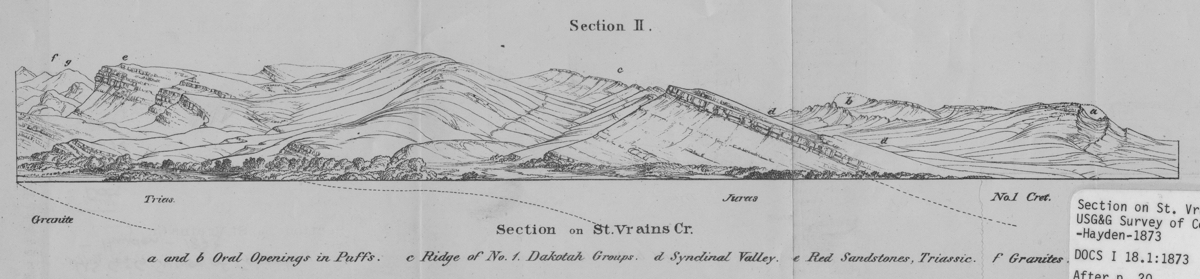
On the east side an oval mass has been removed through the quartzites of No. 1 and the Jurassic group, just exposing the red beds in the bottom of the depression . . . To one traveling between the Hogback ridges and the granites [the] tangential movements of the internal forces do not seem to have disturbed the symmetry of the principal series of ridges.
Annual Report for the 1873 Field Season (Hayden 1874, 28)
Section on St. Vrains [sic] Creek
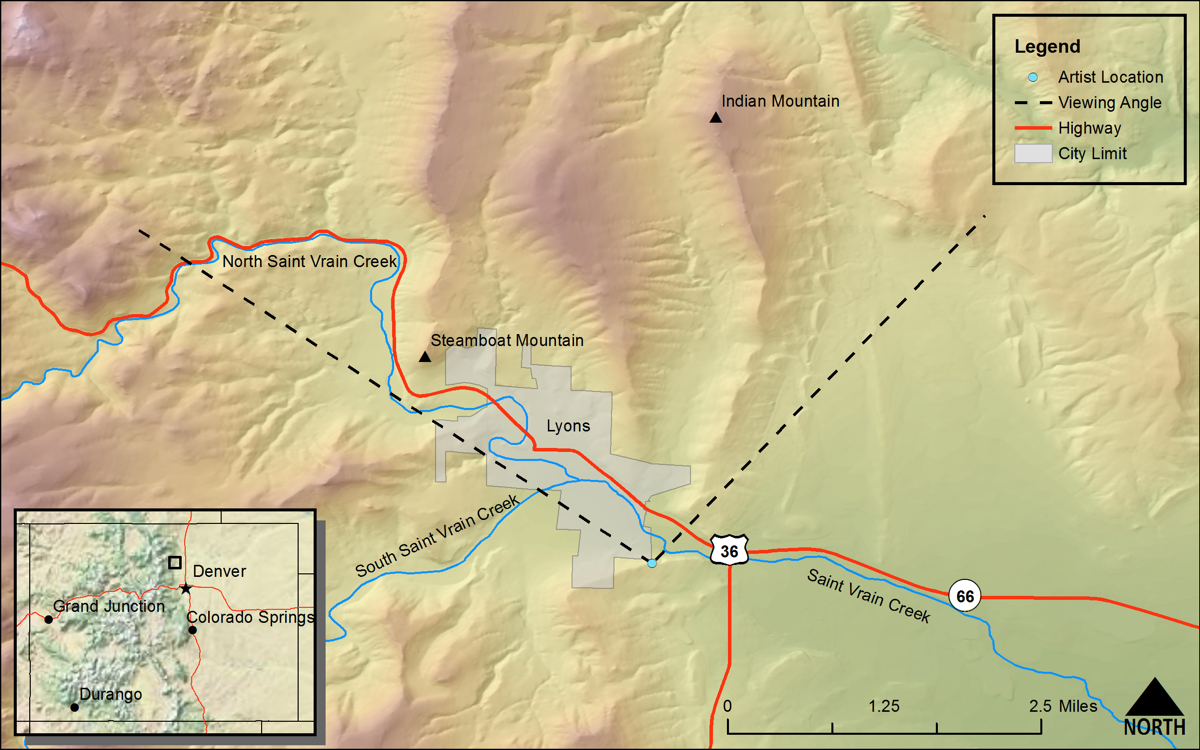
(Annual Report for 1873 Field Season [Hayden 1874], Section II, Section on St. Vrains [sic] Cr.)DRAWN/TAKEN FROM GEOGRAPHIC COORDINATES: LATITUDE: 40°12′39′′ N | LONGITUDE: 105°15′29′′ W | UTM zone 13N | 478,036 mE | 4,450,964 mN
VIEW ANGLE: West-northwest clockwise through north-northeast | COUNTY: Boulder | NEAREST CITY: Lyons
There is a steep ridge just south of South Ledge Ditch from where this drawing/photo was done. It overlooks the town site for the small city of Lyons, which is the location for the Lyons sandstone formation that is well known throughout the Front Range of Colorado.
Many of the drawings in the Annual Reports and the Atlas of the Hayden Survey are nearly exact renderings of what the artist saw on the ground. Some drawings are intentionally altered to enhance a scene in the artist’s eye and to impress the ultimate viewer. This particular drawing of the St. Vrain Valley near Lyons is neither an exact rendering nor an artistically enhanced view; rather, it is drawn from a perspective that is not actually real to more clearly show the rock strata of the scene. When you see the drawing in juxtaposition to the photograph, it is obvious they do not match perfectly. The original artist tilted aspects of the landscape in his eye so that the geology was more easily “read” and understood. The photo could not have been taken from the same place as the sketch, because that place did not exist on the ground. Possibly an oblique aerial photograph could have captured the drawn view with more fidelity, but the photo was taken from the viewing position on the ground that was as true to the drawing as possible.
Probably the most well-known identification with Lyons, Colorado, today actually occurs in Boulder. The unique and quite distinctive architecture and construction material of the buildings of the University of Colorado, Boulder, come from the red rock known by geologists as the Lyons sandstone. The stone was first described and has been long quarried near the small town of Lyons that sits about fifteen miles north of Boulder along St. Vrain Creek. When the Hayden Survey came through the Lyons area (1873), there were only a few scattered settlers along the St. Vrain Valley. The town of Lyons itself was not established until the 1880s.
At the time of the Hayden Survey, St. Vrain Creek and Fort St. Vrain, a few miles out into the plains, were more famous than the sandstone. The fort and creek were named after Ceran St. Vrain and his younger brother Marcellin. They were both contemporaries and sometime business partners of the Bent brothers, who were the early settlers and entrepreneurs; the Bents built and ran Bent’s Fort along the Arkansas River in southern Colorado. The Bents were early settlers, merchants, and eventual politicians along the Santa Fe Trail during the 1830s and 1840s. Ft. St. Vrain was an effort to expand the Bent’s and St. Vrain’s mercantile and supply business to the northern part of the Colorado Territory.
As with most of the major streams and rivers flowing out of the mountains onto the plains, the St. Vrain Valley was also a main gateway into the mountains. Today it is one of two main routes to the city of Estes Park and Rocky Mountain National Park to the northwest. The town that developed at this strategic site is evident in the photo panorama. The city of Lyons today is a vibrant small town that is rapidly becoming an attractive place to live along the booming Front Range. Accelerated growth in the town can be seen in the photograph in the new high school, expanding housing tracts, and infrastructure development that one sees all over town.
The quarries that produce the famous Lyons sandstone are not visible in either the panoramic line drawing done by Hayden’s men or in the photograph. But you can see an outcrop of Lyons on the drawing at (e). The fact of the stone being a foundation for the city’s wealth is obvious as you drive into town from the south or east. All along the road are stone dealers and piles and stacks of the flat, attractive, red rock ready for sale. This is the same red rock seen in other parts of the state, especially in the Gateway Rocks in the Garden of the Gods in Colorado Springs a hundred miles to the south.
The members of the Lyons formation were deposited in the mid-Permian to very early Triassic geologic periods about 250 million years ago. Three distinct members or strata of the Lyons were formed. The upper Lyons is often a whitish to grayish sandstone that is less distinctive than its reddish sibling. It is weaker and is much less attractive than the lower, red member. The middle member is composed of much finer sediment than that which makes up the sandstone of the upper or lower members. It is a shale or claystone that has eroded down to an inconspicuous valley in the landscape. The beautiful red flagstone rock we see in the buildings at Boulder, and frequently in large buildings all along the Front Range, is the lowest member of the formation. It consists of lithified, ancient sand dunes recognized by the distinctive cross-bedded pattern seen in sand dunes around the world today. The coloring comes from the oxidation of the iron in minerals that come from the original rock—this is the same process discussed in the section on the Central Elk Mountains, section 12.
A legitimate question from an inquisitive reader could well be “How do we know that the Lyons sandstone is 250 million years old?” Another, related one, is “How did Hayden know that the Lyons sandstone was Permian in age or even that any given rock formation was of Permian, Jurassic, Cretaceous or any other age?” Understanding these techniques and what Hayden had to work with will be useful in looking at the geologic dating in all of the other sections of this publication. There are two general ways of dating geologic formations. The first and most precise is often called absolute dating—where exact (or nearly exact) dates for a formation can be found through any of several different, yet related, isotope analysis procedures. The second, and the one used by Hayden and all other geologists in the nineteenth century, is generally called relative dating.
Relative dating is based on two characteristics of a formation and is used mostly for sedimentary rocks. The first is the rock’s vertical position in relation to other rocks. With few exceptions a sedimentary rock is initially deposited on top of other, already existing strata. This means that the rock stratum which is on top is younger than the rock below. Geologists call this the “law of superposition.” Therefore, if we know something about the age of the rock below (rock A) and the age of the rock above (rock B) the strata in question, we know that the rock we are dating is between the ages of rocks A and B. In other words we do not know the exact age of our strata, but it is relatively younger than the rock below and relatively older than the rock above—hence we have a “relative age.”
Paleontologists, those specialized geologists who analyze ancient life forms through the study of fossils, have built a sequence of fossils throughout geologic time. They can determine with some accuracy which fossils evolved from others, where certain fossil types sit in the geologic column, and when new life forms began. Even in Hayden’s day, paleontologists knew a great deal about which species of animal, plant, or both preceded or succeeded another. With the law of superposition and a good fossil record, one can determine where a given rock, especially one that contains fossils, fits into the geologic record in a relative sense. No exact dates are known, and in fact, you will not find any exact dates in the volumes and volumes written by the Hayden survey—relative dating was all they had.
Relative dating using fossils is almost always exclusively done with sedimentary rock. Sedimentary rock is the one rock classification where fossil remains of plants and animals can survive the rock making, or lithifying, process. For igneous rocks such as the granites of the Front Range and the volcanic tuffs and lavas of the San Juan Mountains, for example, other dating techniques are needed. What is lost in not being able to read the fossil record in these rocks made from molten magma is more than made up for in the precision of the dates for the rocks obtained from other methods. In fact, these other types of dating techniques are often called “absolute” dating methods (some also call them numerical dating) because they give a precise number of years, plus or minus a margin of error, before the present that the rocks were formed.
Hayden had none of these absolute dating techniques available, because they all depend on scientific advances that occurred much later than his survey. Most of the common absolute dating techniques depend on the half-life of radio isotopes of certain elements. Isotopes are atoms of a given element, such as carbon-14 versus the more common carbon-12, that have the same number of protons as the more abundant atom but more neutrons—in this case carbon-14 has two more neutrons that protons. Many of these isotopes are unstable in nature and disintegrate or decay at a known rate back into the more common atomic state. Scientists use a unit called the “half-life” where one-half of the isotope disintegrates during a known and consistent amount of time. If we know the half-life of an isotope, and if we know how much of the isotope was initially formed, we can test the current material using the constant half-life timeline and calculate with good accuracy how long ago a rock was formed.
One of the most common numerical dating techniques for igneous rock is potassium-argon dating. Potassium-40 is naturally occurring and is found in many common minerals making up igneous rock. It is a radioactive isotope of potassium and decays to argon-40 and calcium-40. Argon is one of the inert elements and remains stable once it is formed. Therefore, if we know how much potassium-40 and argon-40 are in a rock at the present time, with some complex calculations we can determine how many potassium-40 half-lives ago the rock was formed. Potassium-argon dating is ideal for dating older igneous rock because its half-life is 1.3 billion years. To get accurate dates the igneous rock must be at least 50,000 years old—rock younger than this has too minute a quantity of argon to measure precisely. Potassium-argon dating is used extensively for determining the dates of rock formed during Precambrian time—older than about 570 million years. The core rocks of many of the mountains in Colorado are almost all of this Precambrian aged material.
Uranium forms several isotopes that can be dated also. Each of these isotopes has a different half-life. For example uranium-238 has a half-life of 4.468 billion years, uranium-235 has a half-life of 703 million years, and uranium-234 has one of 245 thousand years. Each decays to form specific daughter elements and sometimes continues to decay to form even other daughters. The useful ages of potential dating for the various isotopes range from less than 10,000 years to more than 1 billion years old.
Probably the most well-recognized isotope-dating technique uses carbon-14. Carbon is different from the elements that make up the minerals that create igneous rocks, because the carbon is burned off with the intense heat of any molten magma. Carbon-14 is useful, however, in much nonlithified sediment that contains organic matter. Wood, charcoal, bone, and other organic material can be dated relatively easily using this technique. Organic material gets its carbon from the atmosphere. Photosynthesis uses carbon dioxide to create plant matter. This carbon-based plant matter has a known ratio of carbon-14 to the much more abundant carbon-12. The total carbon in the atmosphere varies somewhat with variations in solar emissions, atmospheric fluctuations, seawater carbon uptake, and other factors. The carbon percentage in the atmosphere for various times in the past has been determined with some precision, so the calibration of carbon can be done with increasing accuracy. The half-life of carbon-14 is close to 5,730 years, which allows us to date organic matter back to about 40,000 years ago.
With increasing scientific expertise, a number of other dating techniques have been developed in the last few decades. They are often complex and use specialized equipment. They give us a wider range of options for dating and confirming dates from other methods. Explanations of these techniques go far beyond the scope of this discussion, but they include procedures like thermoluminescence, fission track dating, cosmogenic dating, amino acid racemization (for fossils), and obsidian hydration. Other precise methods will surely be found in the future.
For this landscape scene the ages of the formations are all well-established using a mix of the above methods. Their relative positions as they are exposed are identical to those same formations found all along the foothills of the Front Range. Fossil finds and other techniques make the dating of all of the strata in the drawing straightforward and quite precise—or as precise as dating anything hundreds of millions of years old can be.

 © 2016 by University Press of Colorado
© 2016 by University Press of Colorado